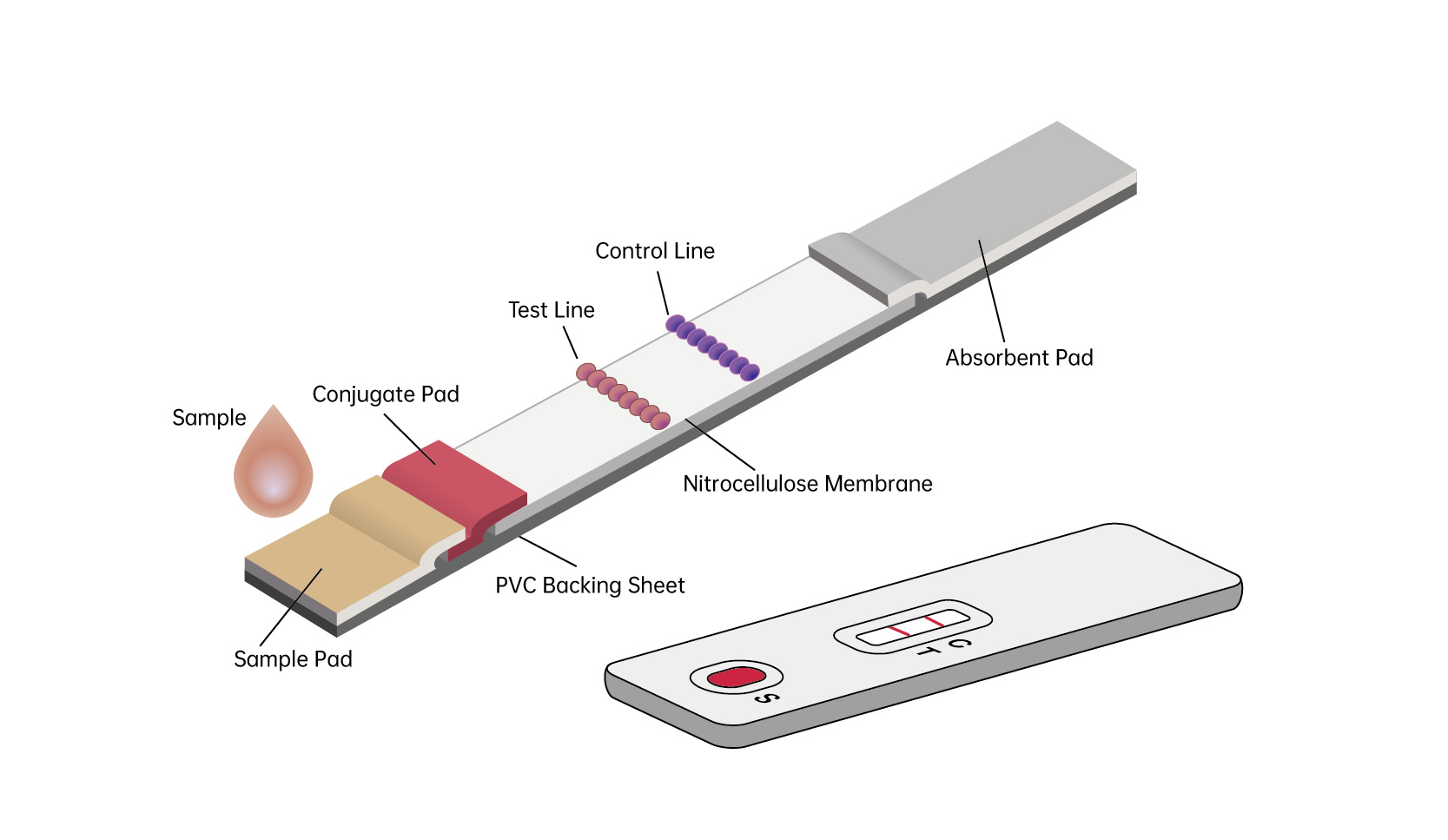
The kit is coated and labeled with HP recombinant antigen (chimed with HP cytotoxin-related gene (cagA) and vacA antigen fragment) using double antigen sandwich and colloidal gold immunoassay technology, as well as colloidal gold immunoassay diagnostic reagent made from other reagents. When the test is performed, if it is a positive sample, the HP antibody in the sample can bind to the colloidal gold-labeled HP antigen, due to the chromatography move forward along the test strip, combine with another HP antigen coated on the nitrocellulose filter membrane to form the gold-labeled antigen ~ anti-HP antibody ~ antigen compound and agglutinate the color. There is a quality control line on the nitrocellulose filter membrane as the control, so when there is a red quality control line and a red reaction line, it is positive. When there is no HP Ab in the sample to be tested, only a red quality control line appear and the result is negative. As for quality control, no matter whether the result is positive or negative, a red quality control line will appear. If there is no red quality control line , the experiment is invalid.