Certificates
Factory Certification
The following is the qualification certificate of our factory: NewScen Coast Bio-Pharmaceutical Co., Ltd.
Click thumbnails to see the details
Product Certification
We can provide the following product certifications to all of our global partner customers.
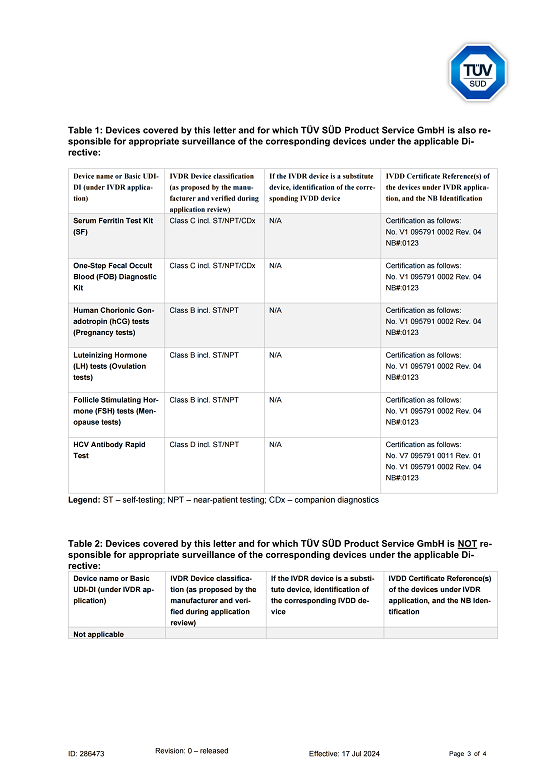
MHRA
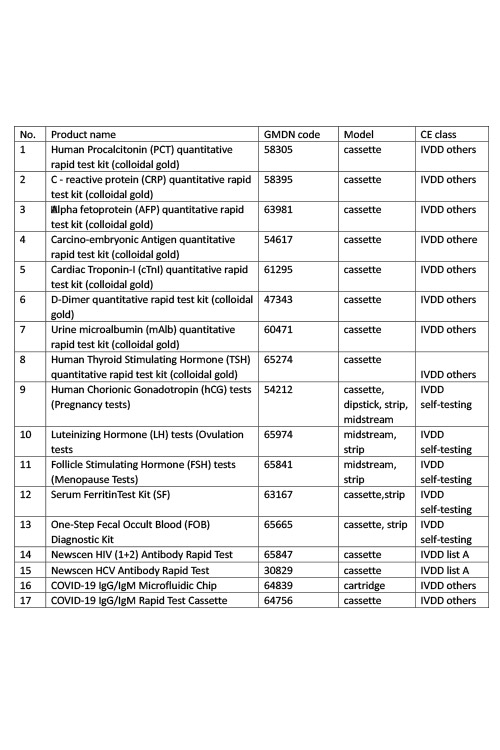
Products: PCT, CRP, AFP, CEA, cTnI, D-Dimer, mAlb, TSH, HCG, LH, FSH, SF, FOB, HIV, HCV
Certificate: CE
Issue Date: 2022/04/28
Expiry Date: Indefinite
Issued By: MHRA
Method: Lateral Flow Assay (Immunochromatography Assay)
RIOMAVIX
Products: MDT, CHIK IgG/IgM, ZIKA IgG/IgM, ZIKA NS1Ag, DEN IgG/IgM, DEN NS1 Ag,
MAR Pf/Pan, HPAb, HPAg, TB, TF, TP
Certificate: CE
Issue Date: 2022/03/08
Expiry Date: Indefinite
Issued By: RIOMAVIX
Method: Lateral Flow Assay (Immunochromatography Assay)
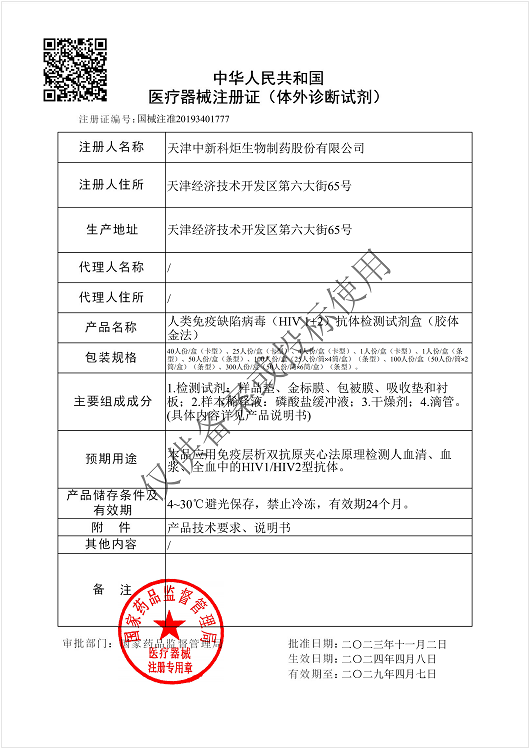
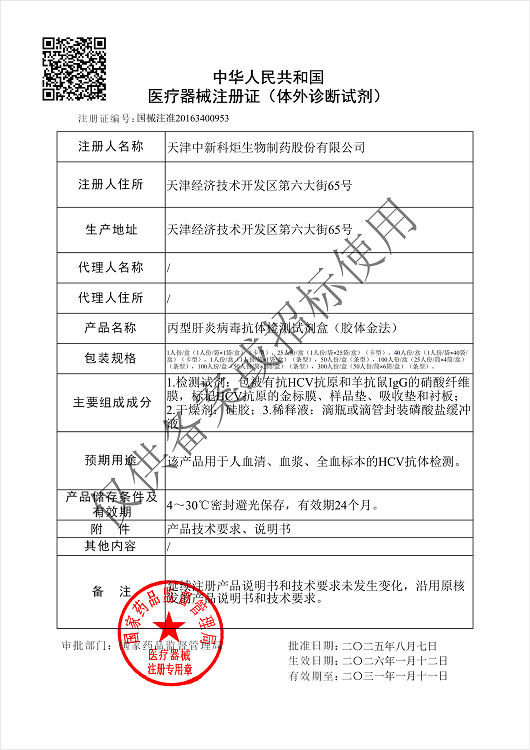
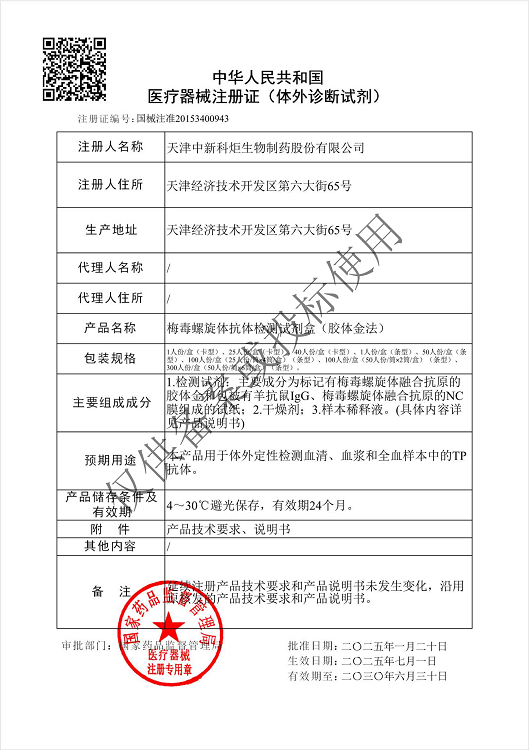
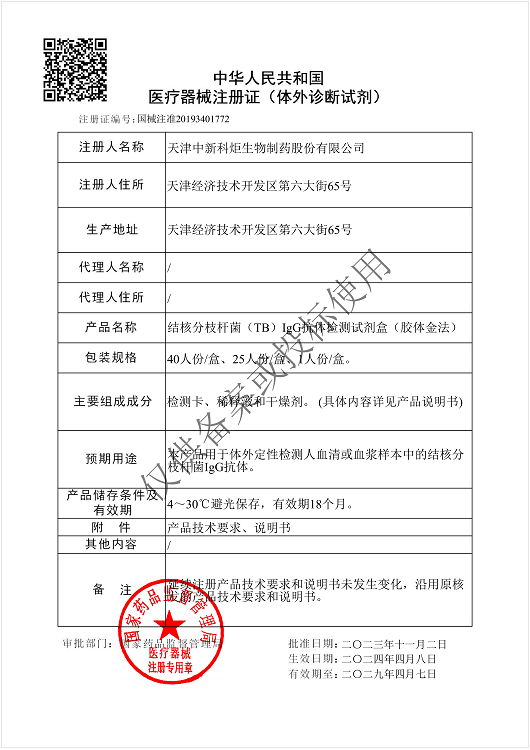
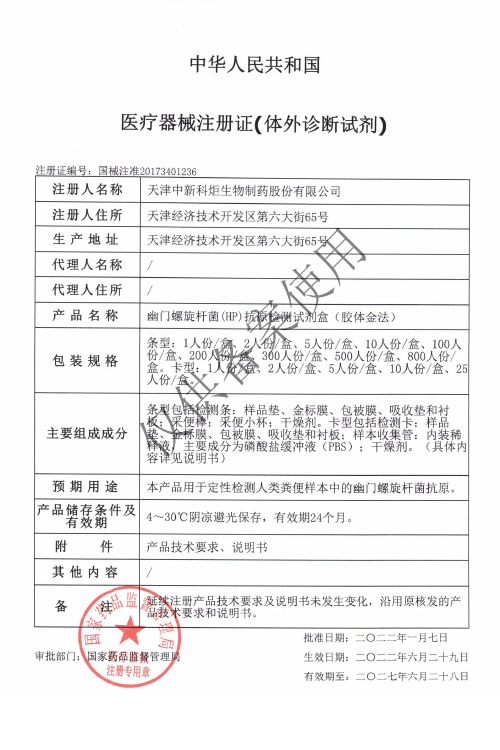
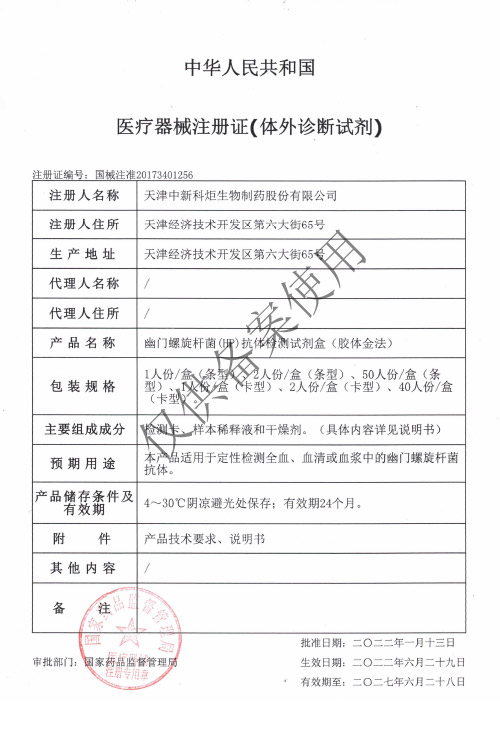
NMPA
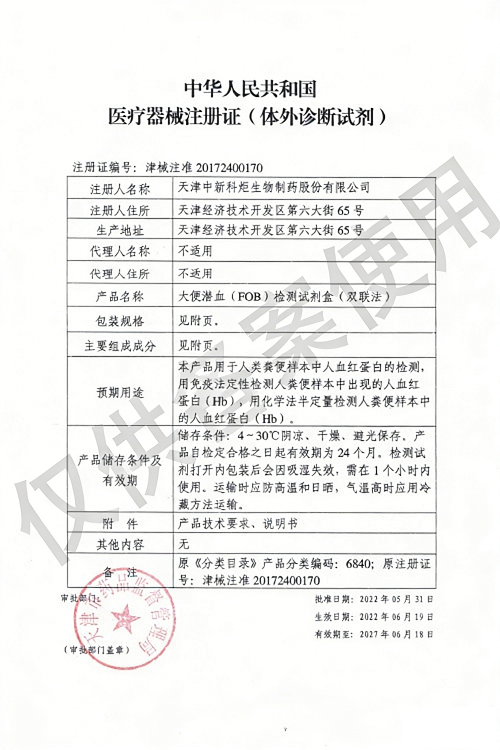
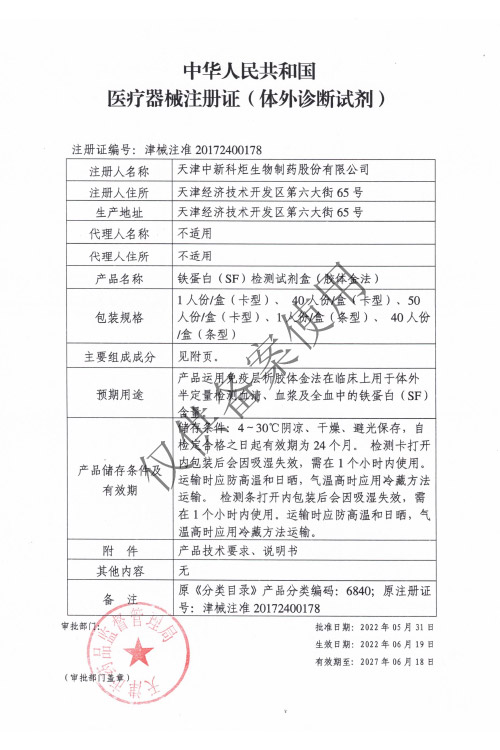
Products: HIV, HCV, TP, TB, HPAg, HPAb, FOB, SF
Certificate: NMPA
Issue Date: HIV 2024/04/08, HCV 2026/01/12, TP 2025/07/01, TB 2024/04/08,
HPAg/HPAb 2022/06/29, FOB/SF 2022/06/19
Expiry Date: HIV 2029/04/07, HCV 2031/01/11, TP 2030/06/30, TB 2029/04/07,
HPAg/HPAb 2027/06/28, FOB/SF 2027/06/18
Issued By: NMPA
Method: Lateral Flow Assay (Immunochromatography Assay)